Collection: Buffers
UFC Bio’s buffer lineup features precision-grade solutions like 0.9% sterile saline for irrigation wash, alongside PBS, TE, TBE, and HEPES, each crafted sterile and USA-manufactured for reliable lab performance.
Free U.S. shipping over $50
-
Sterile Saline Solution, 0.9% - USP Grade
Regular price From $10.00 USDRegular priceUnit price / per -
1X PBS (Phosphate Buffered Saline), 500 mL
Regular price $19.99 USDRegular priceUnit price / per -
1X TE (Tris-EDTA) Buffer, 500 mL
Regular price $29.99 USDRegular priceUnit price / per -
10X PBS (Phosphate Buffered Saline), 500 mL
Regular price $29.99 USDRegular priceUnit price / per -
0.5M HEPES Buffer (pH 7.4-8.0)
Regular price From $34.65 USDRegular priceUnit price / per -
1.0M HEPES Buffer (pH 7.4-8.0)
Regular price From $60.00 USDRegular priceUnit price / per -
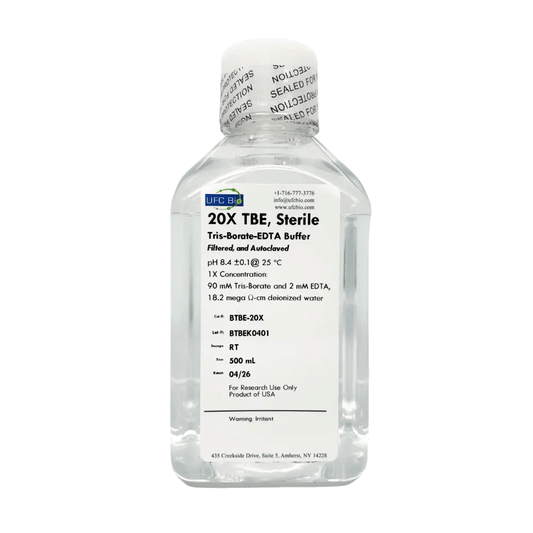
20X TBE (Tris Borate EDTA) Buffer, 500 mL
Regular price $39.99 USDRegular priceUnit price / per -
10X PBS-T (Phosphate Buffered Saline with Tween-20), 500 mL
Regular price $35.00 USDRegular priceUnit price / per -
10X TAE Buffer (Tris Acetate EDTA), 500 mL
Regular price $34.99 USDRegular priceUnit price / per -
50X TAE Buffer - Tris Acetate EDTA Buffer, 500 mL
Regular price $40.00 USDRegular priceUnit price / per -
10X TBE Buffer (Tris Borate EDTA), 500 mL
Regular price $35.00 USDRegular priceUnit price / per -
10X TG-SDS Buffer (Tris-Glycine-SDS), 500 mL
Regular price $34.99 USDRegular priceUnit price / per -
1M Tris Buffer, pH 7.5, 500 mL
Regular price $35.99 USDRegular priceUnit price / per -
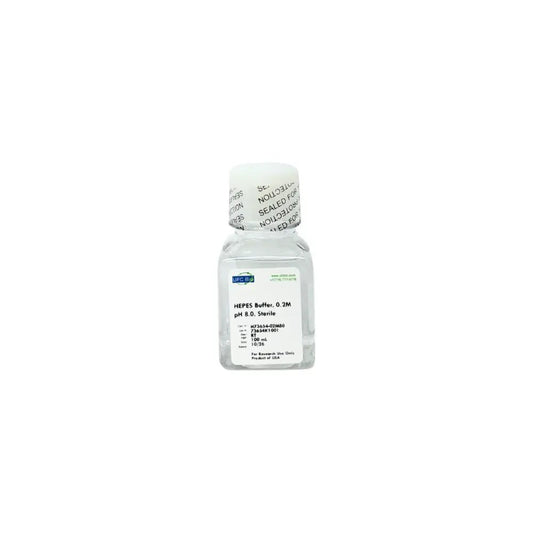
0.2M HEPES Buffer, pH 8.0
Regular price From $54.99 USDRegular priceUnit price / per -
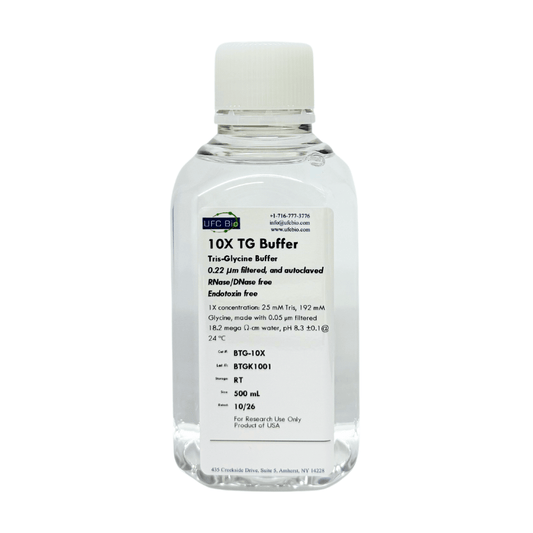
10X Tris-Glycine Buffer, 500mL
Regular price $18.99 USDRegular priceUnit price / per -
TE Buffer, low EDTA, pH 8.0, 500mL
Regular price $40.00 USDRegular priceUnit price / per