UFC BIO Products - Free U.S. shipping over $50
Explore UFC Bio’s top-selling sterile solutions and research essentials — including0.9% sterile saline, USP-grade methylene blue (third-party tested), and a full range of premium buffers, media, and reagents for PCR, DNA sequencing, and other high-precision applications. Proudly made in the USA, every product meets strict quality standards and includes downloadable Certificates of Analysis (CoA) and Safety Data Sheets(SDS).
Free U.S. shipping over $50
-
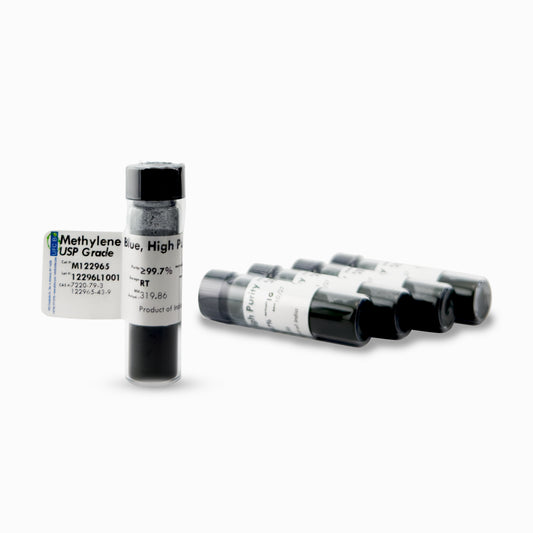
Methylene Blue Powder, USP/Pharma Grade
11 reviews4.91 / 5.0
(11) 11 total reviews
Regular price From $12.50 USDRegular priceUnit price / per -
Methylene Blue USP 1% Solution
23 reviews4.52 / 5.0
(23) 23 total reviews
Regular price $19.50 USDRegular priceUnit price / per -
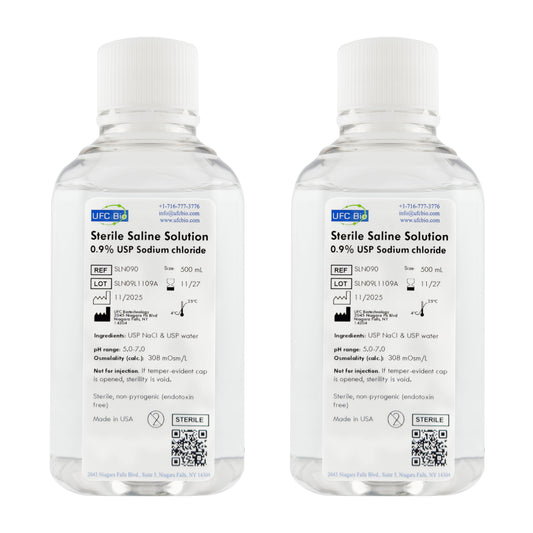
Sterile Saline Solution, 0.9% - USP Grade - RNase/DNase Free
4 reviews5.0 / 5.0
(4) 4 total reviews
Regular price From $10.00 USDRegular priceUnit price / per -
Sterile Water USP – Ultra Pure Grade
Regular price From $9.99 USDRegular priceUnit price / per -
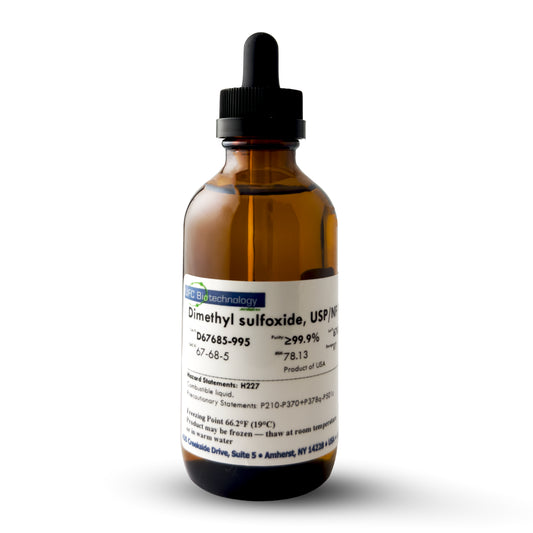
99.9%+ DMSO, USP/NF/ACS Pharma Grade
7 reviews5.0 / 5.0
(7) 7 total reviews
Regular price From $16.00 USDRegular priceUnit price / per -
1X PBS (Phosphate Buffered Saline), 0.20 um filtered, 500 mL
Regular price $19.99 USDRegular priceUnit price / per